
(d) Cr 2O 7 2– + SO 2(g) → Cr 3+ (aq) + SO 4 2– (aq) (in acidic solution)ĭetermine the empirical formula of an oxide of iron which has 69.9% iron and 30.1% dioxygen by mass. (c) H 2O 2 (aq) + Fe 2+ (aq) → Fe 3+ (aq) + H 2O (l) (in acidic solution) (b) MnO 4 – (aq) + SO 2 (g) → Mn 2+ (aq) + HSO 4 – (aq) (in acidic solution)
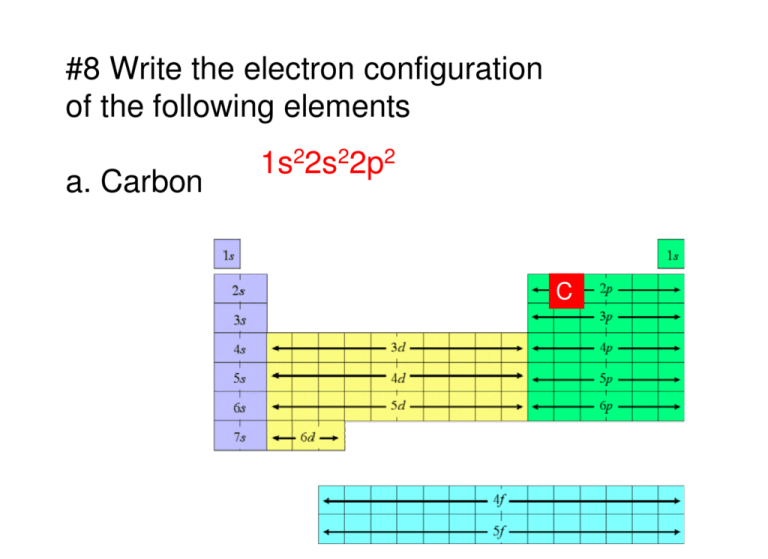
(a) MnO 4 – (aq) + I – (aq) → MnO 2 (s) + I 2(s) (in basic medium) (iii) 2 moles of carbon are burnt in 16 g of dioxygen.Ĭalculate the wavelength of an electron moving with a velocity of 2.05 × 10 7 ms –1.īalance the following redox reactions by ion – electron method : (ii) 1 mole of carbon is burnt in 16 g of dioxygen. is 3.0 × 10 –25 J, calculate its wavelength.Ĭalculate the amount of carbon dioxide that could be produced when The mass of an electron is 9.1 × 10 –31 kg. Thus, its atomic number is 54 + 7 + 2 + 1 = 64. Its electronic configuration is 54 4 f 7 5 d 1 6 s 2. It belongs to group 3 of the periodic table since all f-block elements belong to group 3. It is an f –block element as the last electron occupies the f–orbital. (iii) Since n = 6, the element is present in the 6 th period. Hence, the element is Titanium ( 183d 24s 2. Therefore, it is a 4 th period and 4 th group element. = Number of s–block groups (4s 2) + number of d–block groups (3d 2)
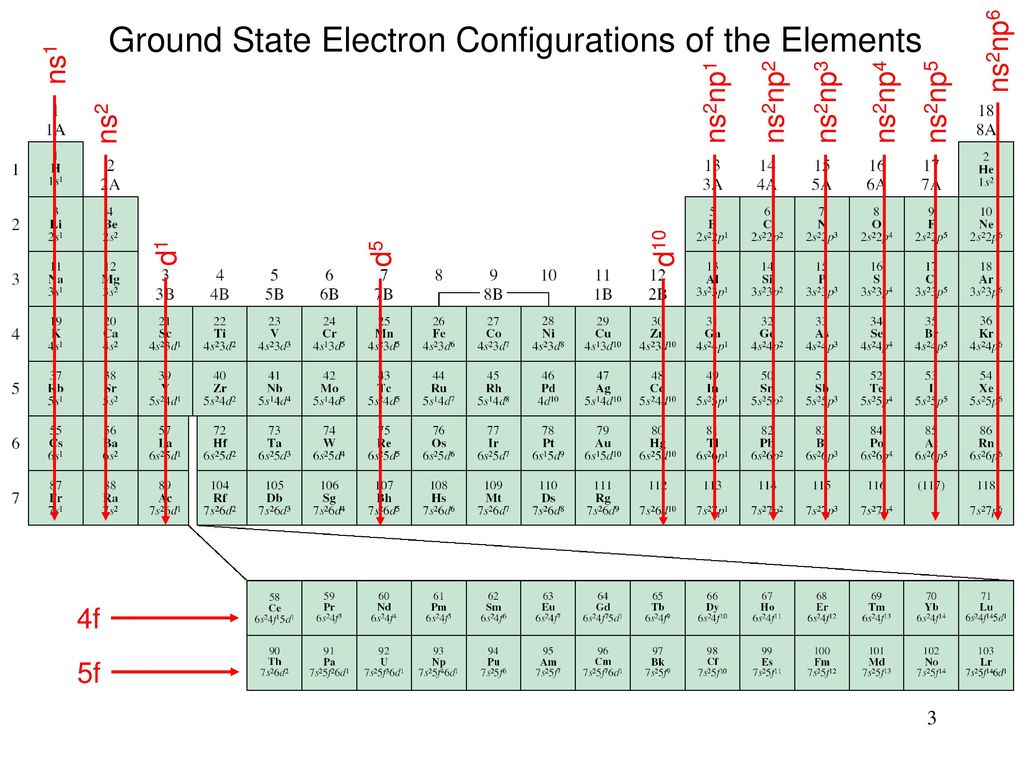
Thus, the corresponding group of the element It is a d–block element as d–orbitals are incompletely filled. (ii) Since n = 4, the element belongs to the 4 th period. Hence, the element is Sulphur ( 103s 23p 4)

Therefore, the element belongs to the 3 rd period and 16 th group of the periodic table. = Number of s–block groups (3s 2) + number of d–block groups ( 10 + number of p–electrons(3p 4)

There are four electrons in the p–orbital. It is a p–block element since the last electron occupies the p–orbital. (i) Since n = 3, the element belongs to the 3 rd period.


 0 kommentar(er)
0 kommentar(er)
